Learn About Metallurgy
Concept Overview
Metallurgy is the process of extracting and refining metals from ores. In this station, we focus on recovering copper from the mineral chrysocolla, using both leaching and electroplating methods.
Engineers simulate this with two major processes:
- Leaching: Dissolving copper into a solution using vinegar.
- Electrowinning (Electroplating): Using electricity to plate pure copper onto a metal surface.

Activity 1: Leaching Copper from Chrysocolla
Materials:
- Crushed chrysocolla, vinegar (5%)
- Coffee filter, funnel, 2 beakers
- Mortar & pestle, gloves, goggles
Place the crushed chrysocolla in a funnel lined with a coffee filter. Slowly pour vinegar over the mineral. After several cycles, the liquid turns blue — a sign that copper has dissolved into the solution, forming copper acetate.
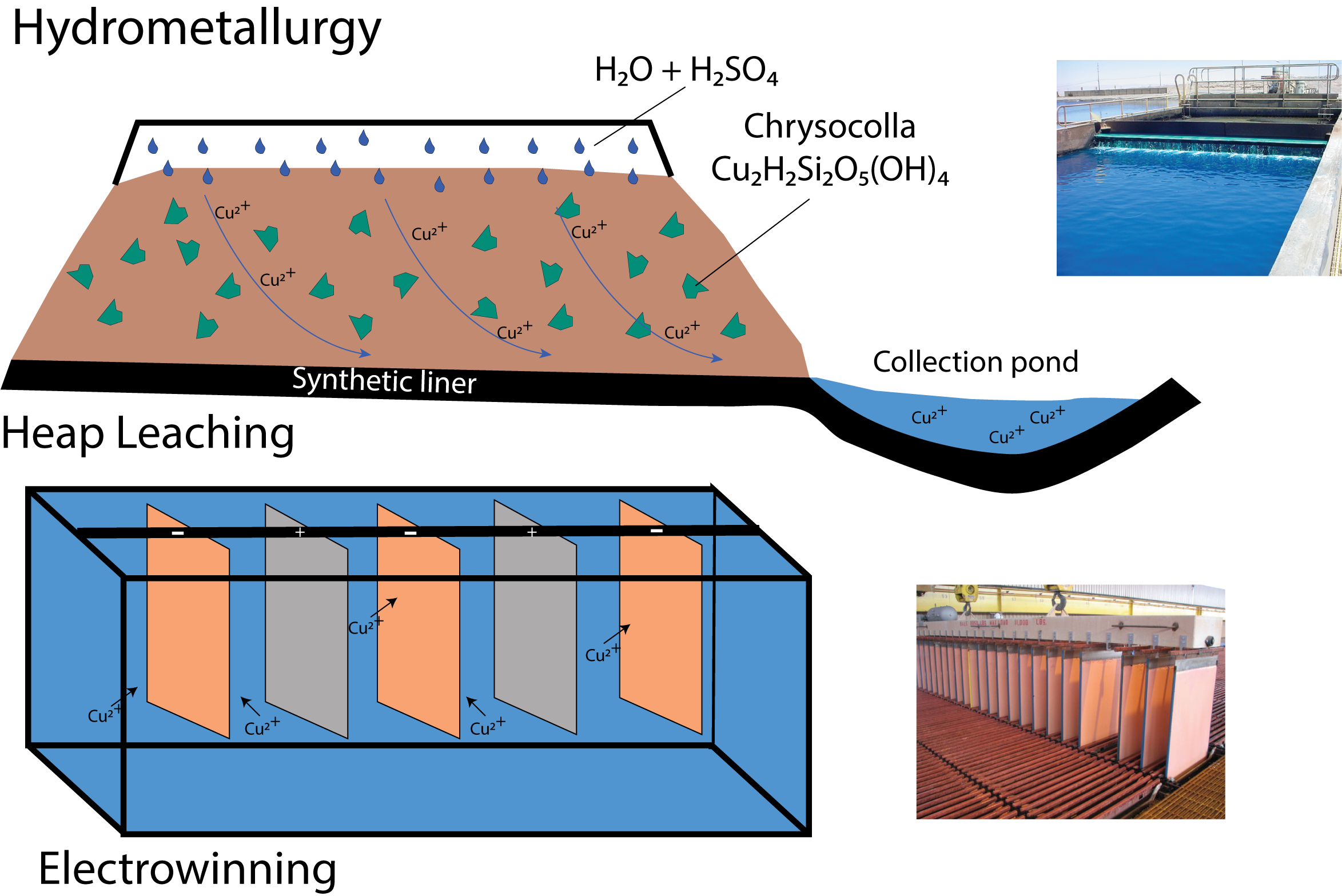
This simulates what happens in a leach pile at a real mine — acid is cycled through the ore to collect copper-rich solution.
Activity 2: Electroplating Copper
Materials:
- Plastic cup, battery, wires & alligator clips
- Nickel (anode), penny (cathode)
- Blue copper solution from leaching
Attach the battery clips to opposite sides of the cup. Connect the red wire to the penny and the black wire to the nickel. When placed in the copper solution and connected to the battery, copper begins to plate onto the nickel.

This shows how electricity is used to recover pure metal from a dissolved state — exactly how copper cathodes are made in the mining industry.
Lab Safety
- Wear goggles and gloves while handling copper solution and vinegar.
- Dispose copper acetate into the correct labeled waste container.
- Use caution when grinding minerals. Stir with the pestle — do not pound.
Scientific Concepts
- Copper oxide minerals dissolve in weak acids like vinegar.
- Electrochemical cells transfer ions using battery-driven charge.
- This process models real-world industrial copper recovery.